DERMABORE+ an internationally patented wound /skin care gel infused with proprietary Cannabinoids
¿Quieres ganar un trabajo como este?
Este cliente recibió 20 diseños de viñeta de 5 diseñadores. Eligieron este diseño de etiqueta de Abdulmumin Imam como el diseño ganador.
Únete gratis Encuentra trabajos de diseñoResumen de Diseño de Etiqueta
The Box desgin attached from Turkey is only as reference and you can use all of hte approved seals on the box but the new product in the US is as follows:
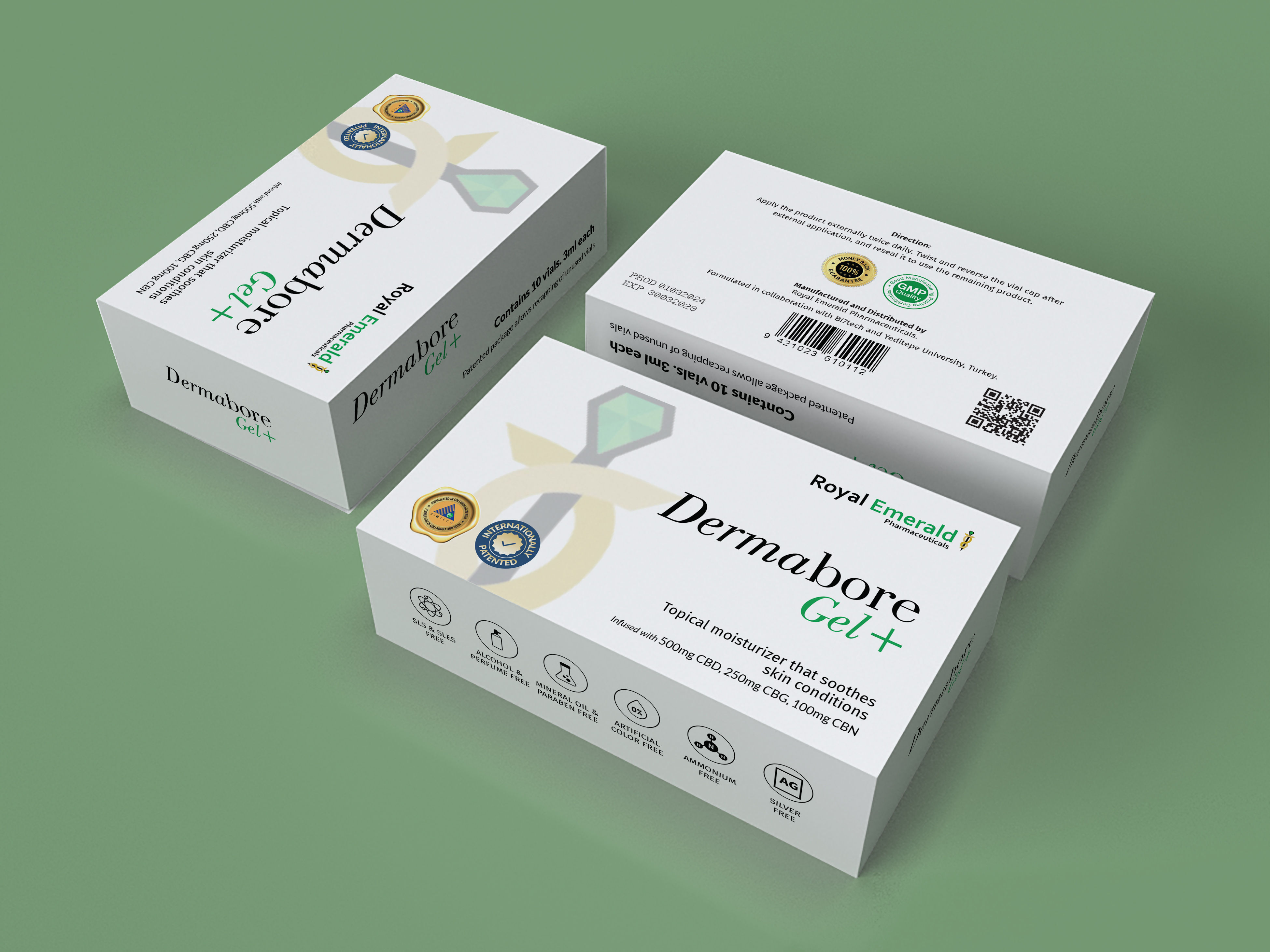
The box dimensions are 5 by 3 by 1.5 inches and it opens from the side.
An instruction page and 10 vials are included inside the box.
Product Details:
Product Name: Dermabore Gel+
Manufactured and tested in a U.S. GMP Facility.
Royal Emerald is licensed by DEA and recognized by FDA as an approved source of cannabinoids for drug manufacturing for FDA approval.
The new and improved product is in the trial phase and not yet approved as a drug by the FDA.
The product is registered by the FDA and internationally patented.
Infused with US-grown and tested cannabinoids.
Claims We Can Make:
Topical moisturizer that soothes skin bruising, rash, scars, and helps with pain and swelling.
Infused with 500mg CBD, 250mg CBG, and 100mg CBN from our proprietary grown genetics of cannabinoids, grown and tested in the US.
Free from SLS & SLES, alcohol & perfume, mineral oil and paraben, artificial color, ammonium, and silver.
FDA registered and internationally patented product.
Manufactured and Distributed by Royal Emerald Pharmaceuticals.
Formulated in collaboration with Bi7tech and Yeditepe University, Turkey.
On the Side of the Box:
Box contains 10 vials of 3ml each.
Patented package allows recapping of unused vials.
Back of the Box:
Direction: Apply the product externally twice daily. Twist and reverse the vial cap after external application, and reseal it to use the remaining product.
Satisfaction guaranteed or your money back.
Inside the box cover shall state the money-back guarantee: "Mail the unused product, complete the survey in the packaging, and return it back to the manufacturer with the original packaging within 30 days of purchase for a full refund or credit."
Additional Requirements:
The box must have space for expiration date and lot number.
The box must have room for a barcode and QR code.
Objetivo del mercado(s)
Wound care, diabetics, cancer patients, cruise lines, military , first responders, post surgery, skin care, alergic reactions
Tipo de industria / entidad
Pharmaceuticals
Colores
Colores seleccionados por el cliente para ser utilizados en el diseño del logotipo:
Mira y siente
Cada control deslizante ilustra las características de la marca del cliente y el estilo que debe comunicar el diseño de tu logotipo.
Elegante
Atrevido
Juguetón
Serio
Tradicional
Moderno
Atractivo
Profesional
Femenino
Masculino
Vistoso
Conservador
Económico
De Alta Gama
Requisitos
Debes tener
- an area for lot number and expiration date. stamp for GMP, Patented, registered trademark,
Agradable de tener
- QR and Bar code must be on the box
No debería tener
- Any claims that it is FDA approved,